
CLATHRATE HYDRATES
Our research focus on the study of clathrate hydrates, from the SCIENCE (fundamental) to the ENGINEERING (applied), so that we can further our understanding of their properties and develop solutions for their application.
Clathrates hydrates are crystalline inclusion structures formed from the hydrogen bonding of water molecules (host) arranged to form cavities that enclose small molecules (guest). These cavities will typically accommodate one guest molecule and the crystal structure formed is largely dependent on the size of the guest molecule(s).
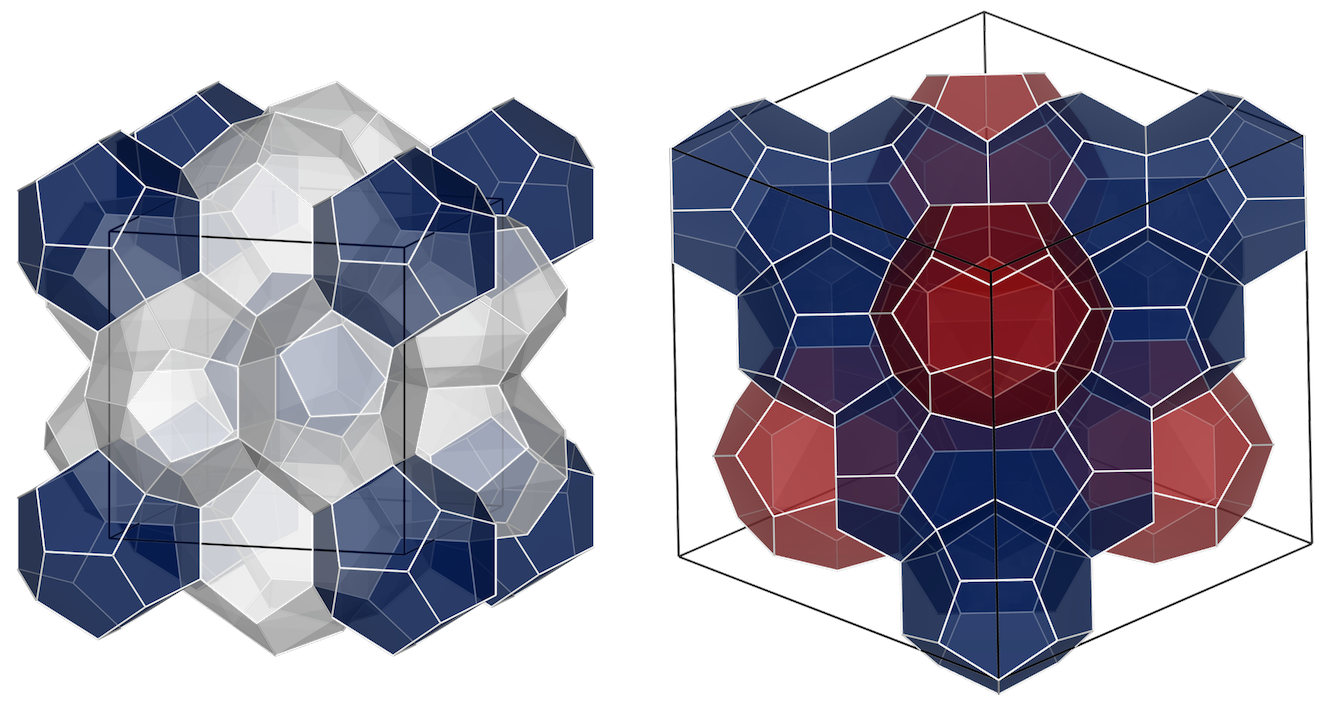
Type I unit cell is composed of two 512 cavities (blue) and six 51262 cavities (gray), whereas the Type II unit cell has sixteen 512 cavities (blue) and eight 51264 cavities (red).
Clathrate hydrates, or simply hydrates, are fascinating structures as they are mainly composed of water (at least 85%), and hydrates formed of gases contain a high density of gas, as most of the cavities can be occupied by a gas molecule. One volume of hydrates contains about 160 times the volume of gas at STP. Hydrates are a solid solution where water and gas “mix” in the ratio of about 6:1. Gas hydrates are typically stable at high pressures and low temperatures, conditions which depend on the gas(es) forming the hydrates.
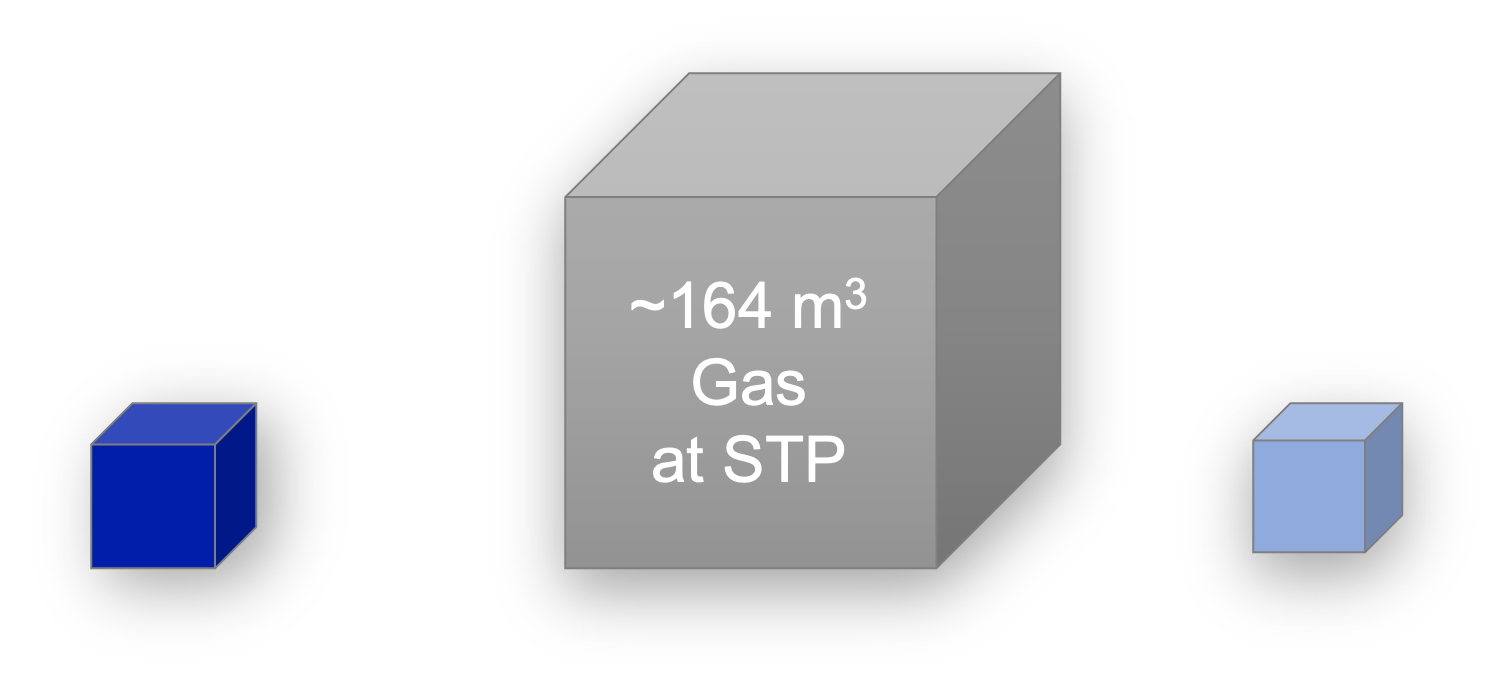
Illustrative volume of gas contained in one volume of hydrates.
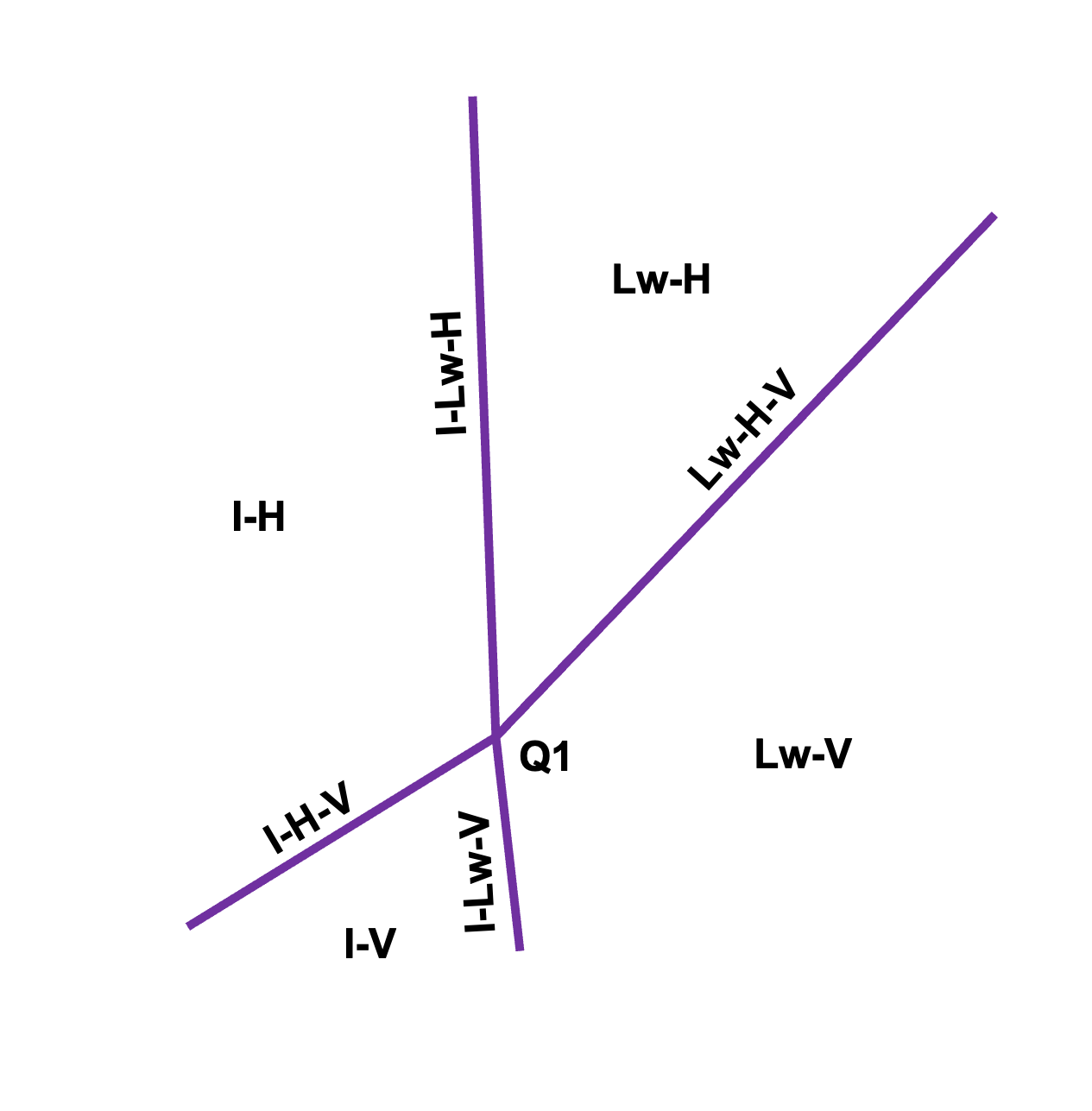
Illustrative phase diagram for gas hydrates. Lines correspond to three-phase equilibrium, separating the two-phase regions. The label correspond to liquid water (Lw), ice (I), hydrate (H), and vapor (V). The intersection of the three-phase lines correspond to the quadruple point (Q1), where I-Lw-H-V coexist.
Chemical & Biological Engineering Department | Colorado School of Mines | Golden, Colorado 80401
© 2025 P2F Lab